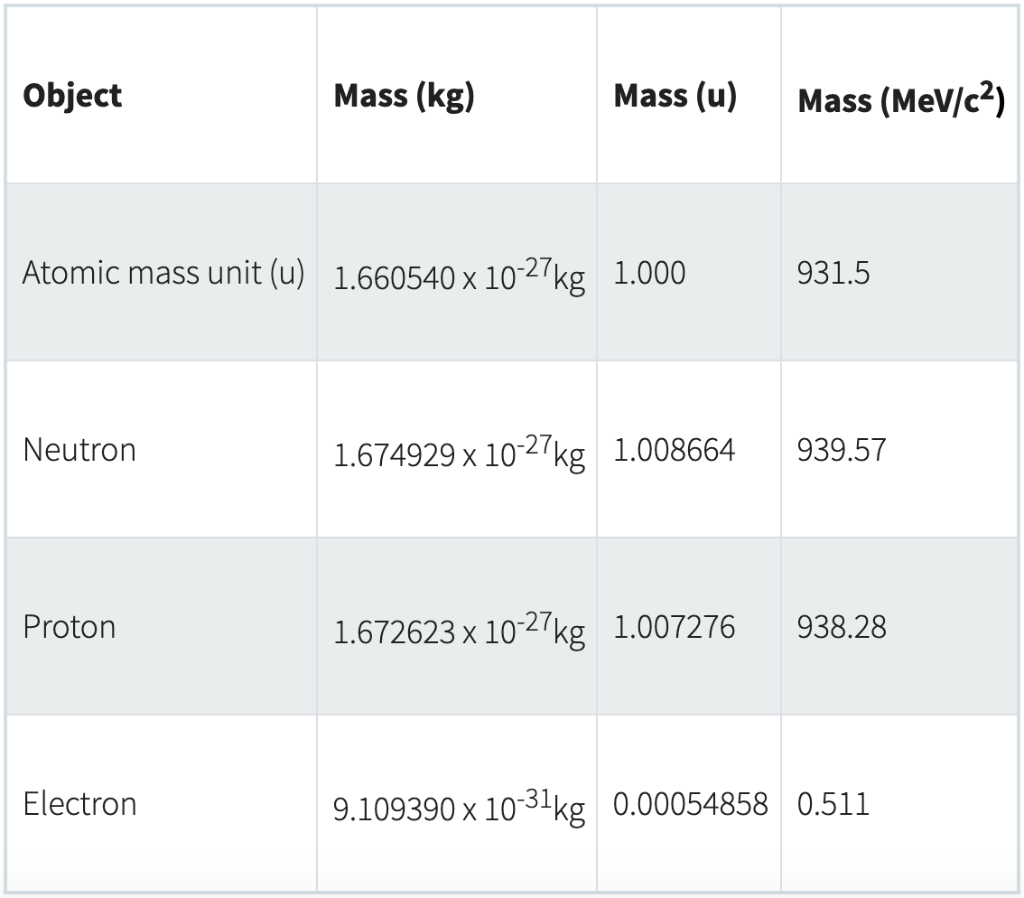
electron mass: Numerical value: 9.109 383 7015 x 10-31 kg : Standard uncertainty: 0.000 000 0028 x 10-31 kg : Relative standard uncertainty: 3.0 x 10-10: Concise form 9.109 383 7015(28) x 10-31 kg : Click here for correlation coefficient of this constant with other constants. Atomic mass unit (or "amu") - also known as "u", or "unified atomic mass unit". ★ u = 1.660540*10^-27kg (I know. what a number!). speaking it's going to be the mass of a proton 'cause the mass of a proton's going to be so much larger than the mass of an electron. And so you would expect that its mass is approximately one unified atomic.

Electron Mass
In Plain English Why Neutrino Mass and Oscillation Won A Nobel Prize. Science 2.0

Nitrogen Big On Periodic Table Of The Elements With Atomic Number Images and Photos finder
PPT Atoms and Nuclear Chemistry PowerPoint Presentation, free download ID1094179

Question Video Calculating the Atomic Mass of a Fluorine19 Atom in Unified Atomic Mass Units
The mass of an electron can be expressed as (A) 0.512 MeV (B) 8.19 × 1014J/c2 (C) 9.1 × 1031
Atomic Mass — Definition & AMU Expii

The mass of electron in atomic mass unit is

3 Ways to Calculate Atomic Mass wikiHow Chemistry Class 12, Chemistry Classroom, Teaching
Periodic Table Number Of Protons
Electron Structure презентация онлайн
Gcse Periodic Table With Mass And Atomic Numbers Matttroy
Basic Nuc Physics

(PPTX) Anatomy of an Atom Parts of an Atom Nucleus (positive, mass of 1 amu) Neutron (, mass of

Atomic Mass Of Mercury 7 Images Periodic Table With Names And Atomic Mass Number, Atomic
38+ how to calculate the mass of a proton RakeshTosia

PPT Aim How can we explain Einstein’s energymass relationship? PowerPoint Presentation ID
how to calculate mass of electron in amu
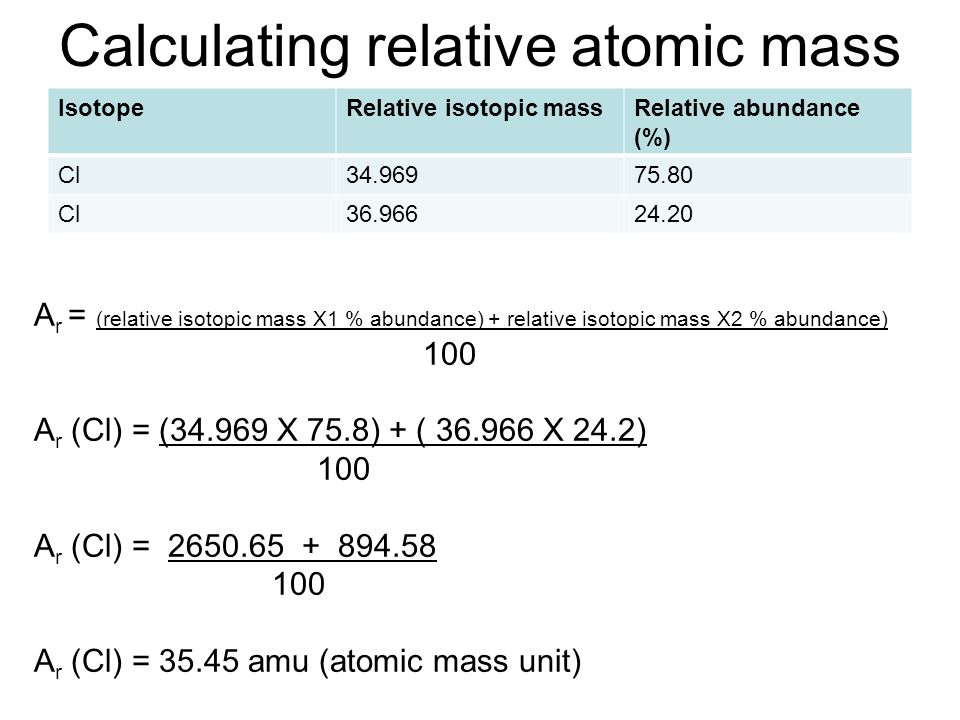
how to find the average atomic mass Hunter Turninaing
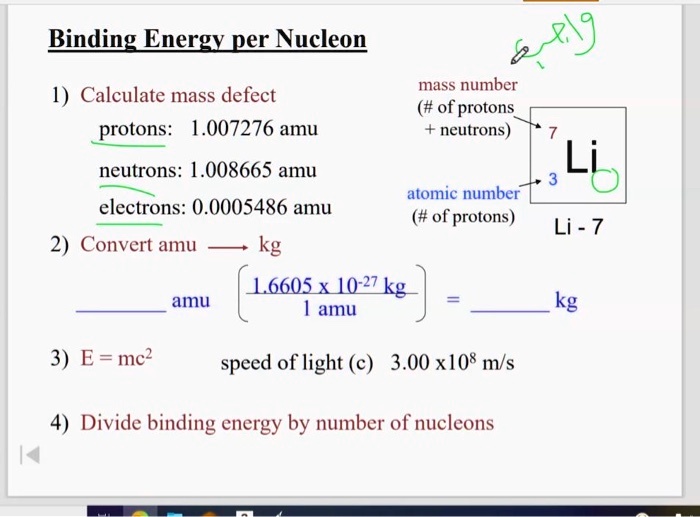
SOLVED Binding Energy perNucleod mass number ( of protons neutrons) 1) Calculate mass defect
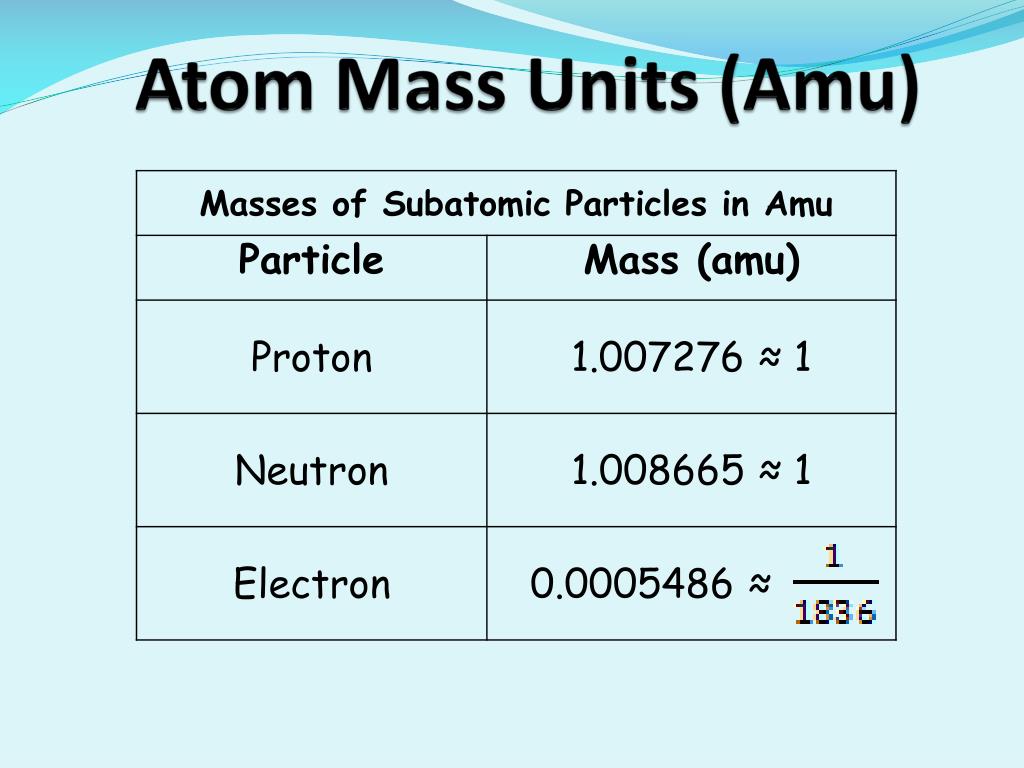
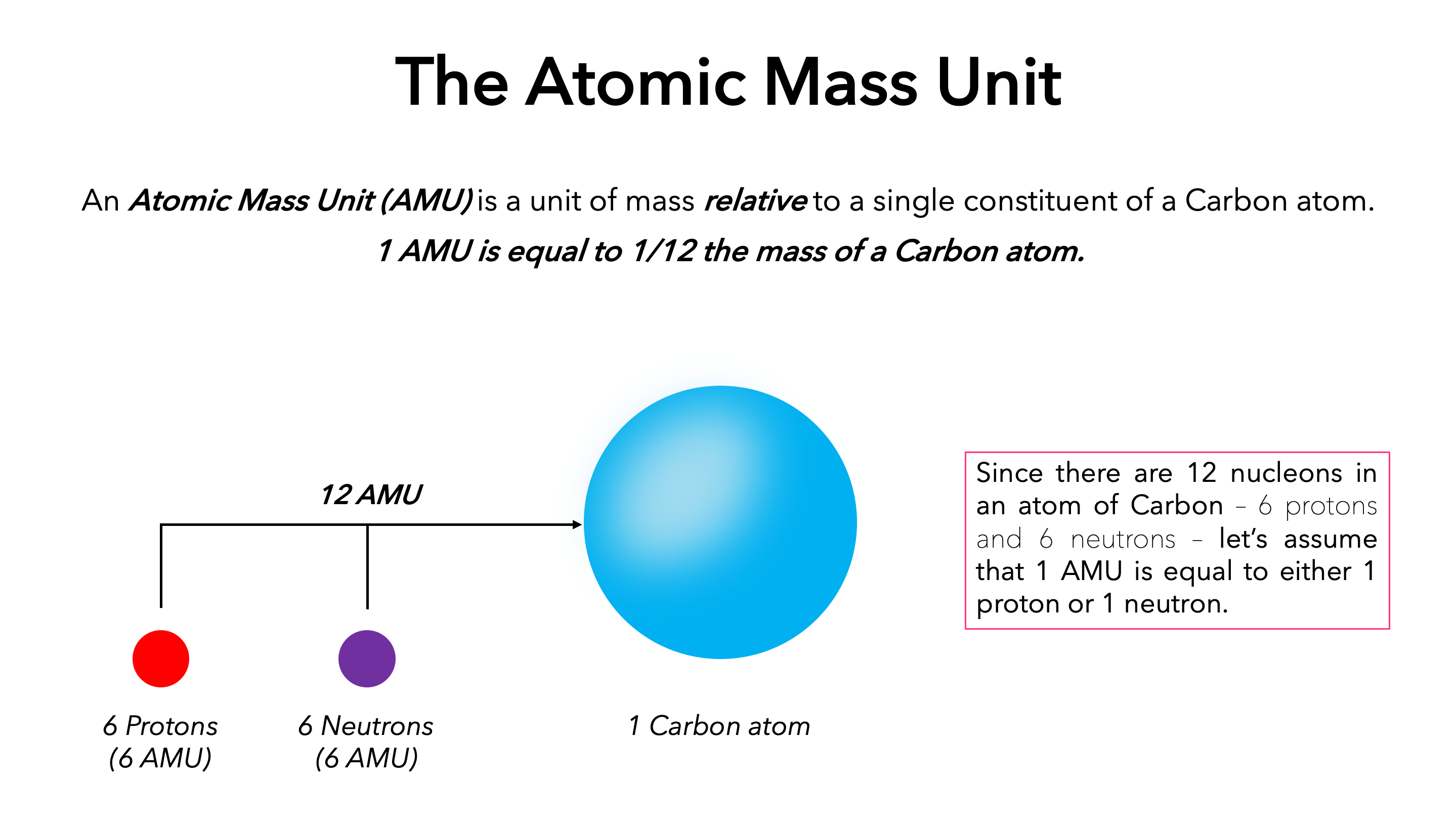
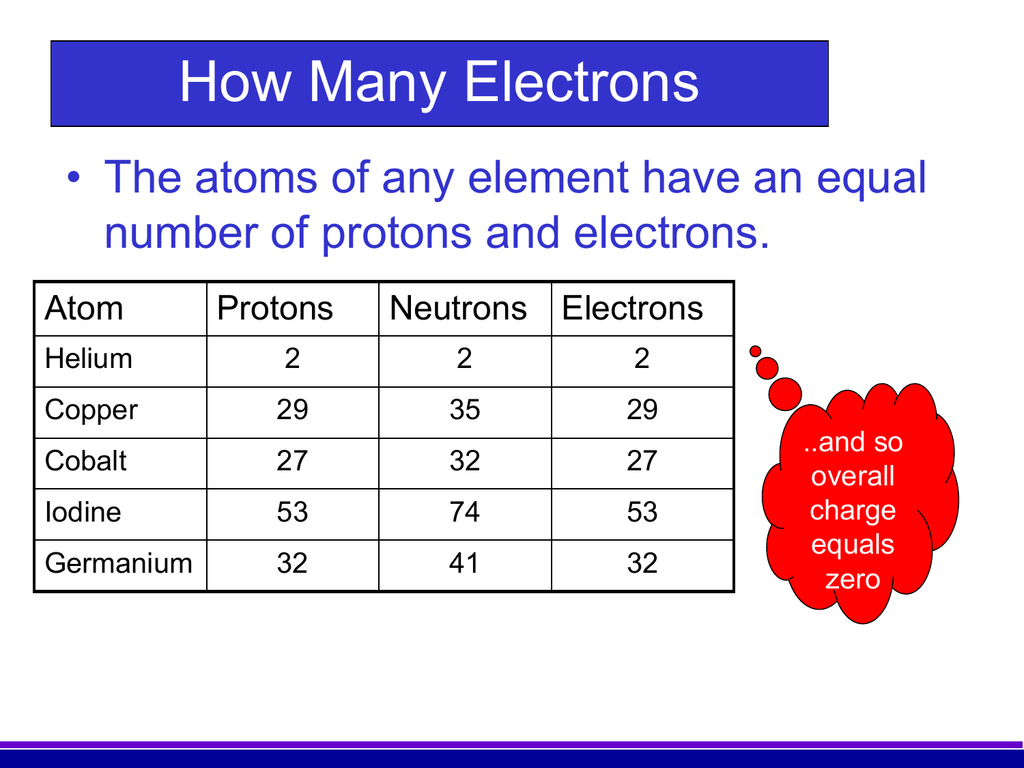
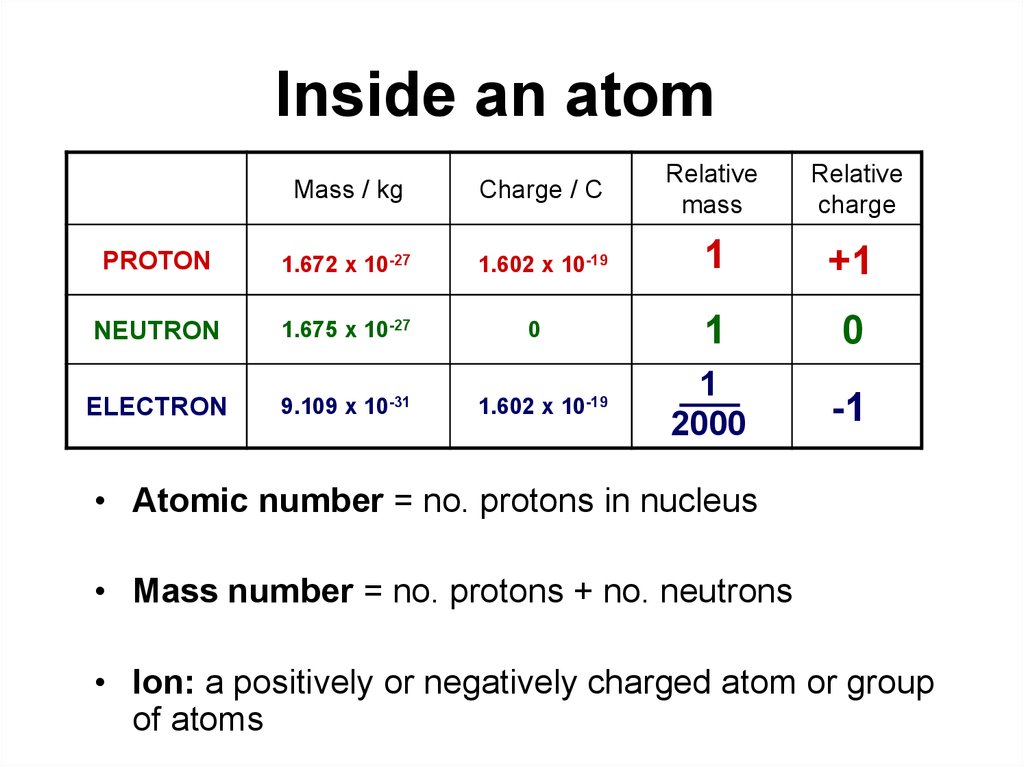
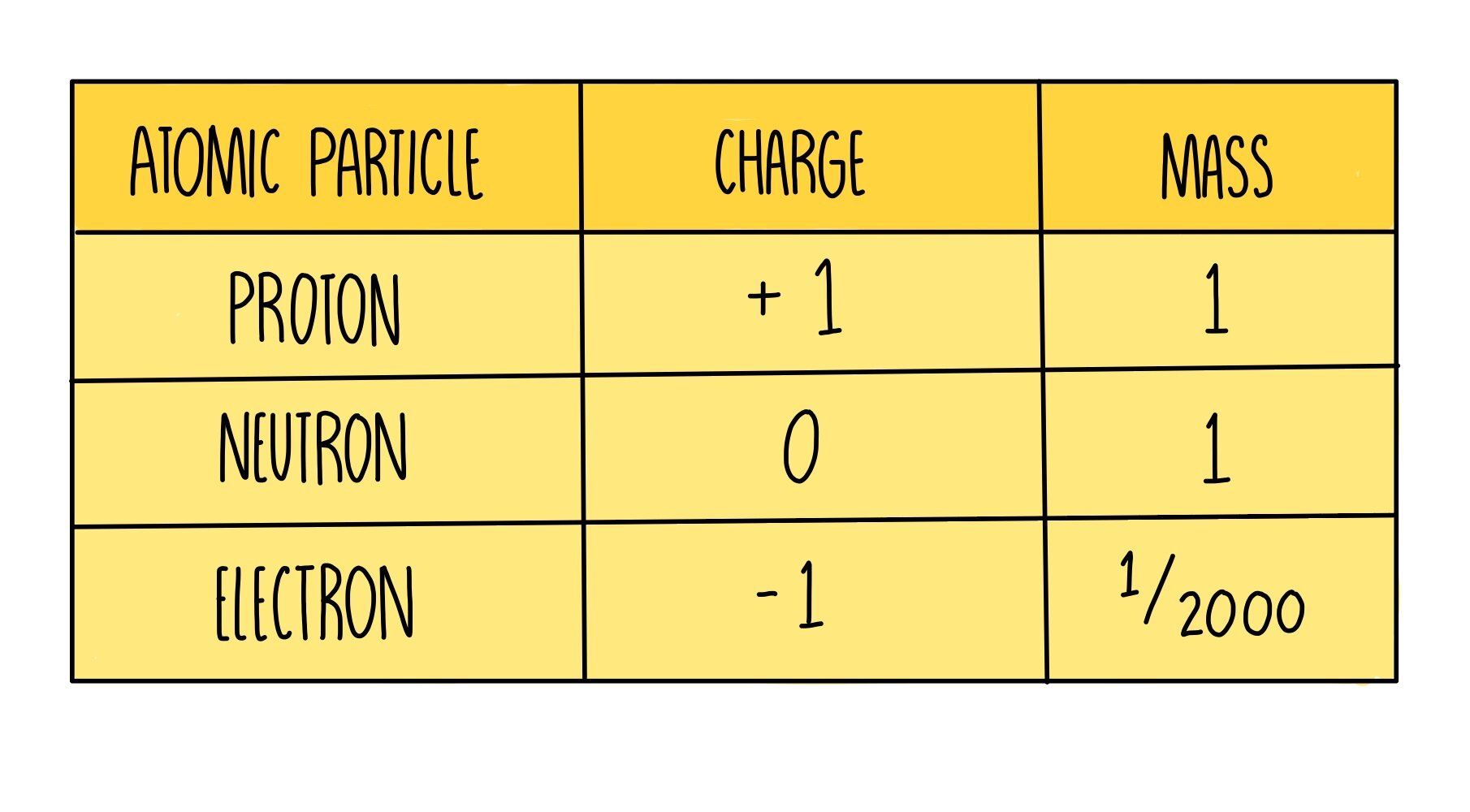
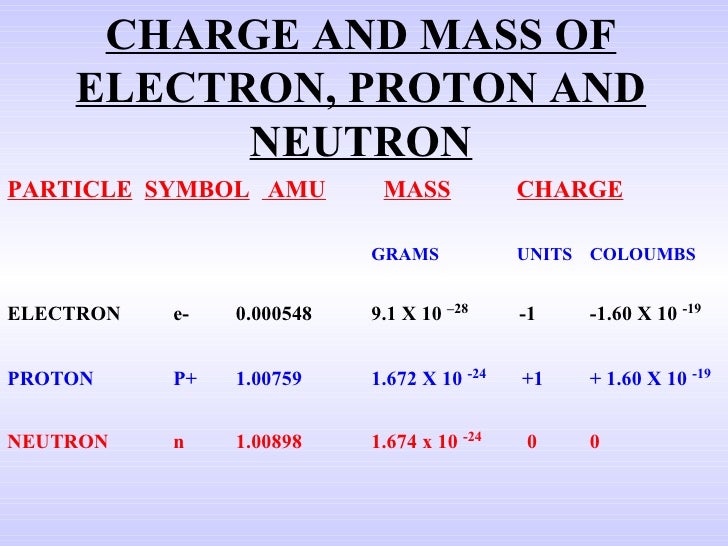
Electron: It is a subatomic particle and is a negatively charged species. The symbol of an electron is e-. Mass of electron in amu (atomic mass unit): The mass of an electron in amu is determined by the mass of an electron (in Kg) divided by the mass of 1 amu. Mass of electron = 9. 109 × 10-31 Kg; Mass of 1 amu = 1. 66 × 10-27 Kg. The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. Protons are particles with a charge of 1+ and a mass of 1.0073 amu. Neutrons are particles with no charge and a mass of 1.0087 amu. Electrons are particles with a charge of 1− and a mass of 0.00055 amu.